Fotos / Sonidos
Observ.
janetwrightDescripción
On wet rock wall with Sphagnum. Shoot width 0.9mm, leaf 0.4 x 0.4mm.
Fotos / Sonidos
Observ.
botanicum-vitaeDescripción
Detailed description here:
http://www.efloras.org/florataxon.aspx?flora_id=50&taxon_id=250062431
Qué
Lince Americano (Lynx rufus)Observ.
cswoodDescripción
CSW Yard Woodbury Connecticut, Jul 2024. © C.S. Wood
Qué
Cuclillo de Pico Amarillo (Coccyzus americanus)Observ.
kristofzDescripción
window kill at Yale University Kline Tower (SW corner) found by Carol Hwang
Fotos / Sonidos
Observ.
malisaspringDescripción
At Hoffman's Water X Scapes Garden Center. I'm thinking maybe Golden Winged or Needham's? This is the same spot Rambur's forktail was found... Specimen was captured with permission.
Fotos / Sonidos
Observ.
peptolabDescripción
Jenningsia macrostoma (Ekebom et al., 1996) Lee, Blackmore and Patterson 1999 from the northernmost saprobic edge benthos of the spring-fed freshwater coastal pond at Ocean Dunes Apartments in the Atlantic Double Dunes Preserve. The sampling site is just 200 meters from the edge of the Atlantic Ocean.
Imaged in Nomarski DIC on Olympus BH2S using SPlanapo 20 0.70, Splanapo 40 0.95, and SPlan 100 1.25 oil objectives plus variable phone cropping on Samsung Galaxy S9+.
The cells are highly metabolic and measure up to 130 um when fully extended. There are fine pellicular striations in an s-helix configuration. There is a single thick emergent flagellum. Two large ingestion apparatus rods are apparent along with a curved refractile cytoskeletal arc arising from the right rod and curving towards the anterior of the cell. There is a large ellipsoid macronucleus with several large nucleoli in the posterior part of the cell.
"Jenningsia macrostoma (Ekebom et al., 1996) Lee, Blackmore and Patterson, n. comb. This species has been reported with lengths from about 64 to 114 µm. The body is anteriorly narrowed and posteriorly rounded. It is very metabolic and has fine pellicular striations following a S-helix. The flagellar pocket is situated on the left ventral face of the cell. Two flagella are seen in the flagellar pocket, but only one flagellum emerges. The flagellum is slightly shorter than the cell and beats freely. The ingestion apparatus with two well marked rods is strongly developed. A refractile cytoskeletal arc arises from the right rod and curves towards the anterior of the cell. Optical sections through the anterior part of the cell show this as a short curving structure extending from near the front pole of the cell to near the anterior end of the ingestion rods. The nucleus is situated in the posterior part of the cell. Refractile granules are randomly distributed inside the cell. Rod-shaped muciferous bodies lie alongside pellicluar striations. Cells glide with a squirming movement. Less common than J. fusiforme" (1).
"This species has been described as Peranema macrostoma from marine sites at tropical and subtropical Australia, Brazil (Larsen and Patterson, 1990; Ekebom et al., 1996; Lee and Patterson, 1999). Like Peranema fusiforme, it has been known that P. macrostoma has a short, curved recurrent flagellum which is tightly pressed to the cell surface (Ekebom et al., 1996). Figure 4h was taken from Ekebom et al. (1996; Fig. 4e). In this figure, the recurrent flagellum was indicated by an arrow, but the arrow indicates the curving element of the intracellular cytoskeleton. Only one flagellum emerges from the anterior opening canal as there is no recurrent flagellum emerging from the slit-like opening of the flagellar pocket (Fig. 4k)" (1).
"Jenningsia and Peranemopsis were created by Schaeffer (1918) and Lackey (1940) respectively to contain peranemid flagellates having one emergent flagellum and flexible body with pellicular striations. They are distinguished from the genus Peranema by having one flagellum, other species having a recurrent flagellum lying in a groove on the ventral face of the body (Fig. 4g). Peranema fusiforme and P. macrostoma have been described with a short, curved recurrent flagellum which is tightly pressed to the cell surface (Larsen, 1987; Larsen and Patterson, 1990; Ekebom et al., 1996). Figure 4a of Peranema fusiforme was taken from Larsen and Patterson (Larsen and Patterson 1990; Fig. 24e) and figure 4h is of Peranema macrostoma and from Ekebom et al. (Ekebom et al., 1996; Fig. 4e), respectively. In these figures, the structure that was interpreted as the recurrent flagellum was indicated by arrows. Our present observations of both species indicate that the structure is a previously undescribed element of the cytoskeleton associated with the ingestion apparatus. As seen in figures 4d and 4k, only one flagellum emerges from the anterior opening canal (arrows). We are therefore of the view that both of these species lack an emerging recurrent flagellum. As the genus Jenningsia was described and distinguished from Peranema on the basis of the absence of the second flagellum, we believe these two species are most appropriately transferred to that genus. Peranemopsis was described by Lackey (Lackey, 1940) also as a peranemid with a single emergent flagellum. It was described without reference to Jenningsia" (1).
- Australian records of two lesser known genera of heterotrophic euglenids – Chasmostoma Massart, 1920 and Jenningsia Schaeffer, 1918 W.J. Lee, R. Blackmore and D.J. Patterson. Protistology 1, 10-16 (1999).
Observ.
brandoncorderDescripción
Collected in eutrophic pond water among floating aquatic plants and surface water. 8/3/2024, Dane County, Wisconsin.
Observ.
brandoncorderDescripción
Collected in eutrophic pond water among floating aquatic plants and surface water. 8/3/2024, Dane County, Wisconsin.
Observ.
brandoncorderDescripción
Collected in eutrophic pond water among floating aquatic plants and surface water. 8/3/2024, Dane County, Wisconsin.
Observ.
laneallenDescripción
Scale Bar = 10 µm
This taxon should be updated to reflect that the genus Rhopalodia is now subsumed into the genus Epithemia.
Fotos / Sonidos
Qué
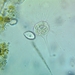
Filo CercozoaObserv.
crseaquistDescripción
Water sample taken from the edge of a freshwater playa on 2023-12-07 using a turkey baster.
The images show the flattened ovoid shape of the test with a lens like cross section. No rod like spines are visible. There do appear to be xenosomes. The third image seems to show a CV and nucleus. It seems to be between 30 and 40 microns long.
Videos:
https://youtu.be/rNY33wLxCXo
https://youtu.be/BZW5JillJPo
Observ.
crseaquistDescripción
Water sample (freshwater) was taken on 07/20/2024 using a turkey baster.
Fotos / Sonidos
Observ.
crseaquistDescripción
Water sample (freshwater) was taken on 07/20/2024 using a turkey baster.
Fotos / Sonidos
Observ.
crseaquistDescripción
Water sample (freshwater) was taken on 07/20/2024 using a turkey baster.
Observ.
brandoncorderDescripción
Collected from floating aquatic masses in wetland among emergent plants. Dodge County, Wisconsin. 7/14/2024.
Fotos / Sonidos
Observ.
trientalidDescripción
Catalogue of Microscopic Organisms of the Everglades Part 2 p.187
Fotos / Sonidos
Observ.
trientalidDescripción
Catalogue of Microscopic Organisms of the Everglades Part 2 p.187
Qué
Colibrí Garganta Rubí (Archilochus colubris)Observ.
rickcechDescripción
Ruby-throated Hummingbird
Qué
Piranga Escarlata (Piranga olivacea)Observ.
altspenDescripción
Bird banding breakfast event. First male Scarlet Tanager at the nets!
Fotos / Sonidos
Observ.
peptolabDescripción
Spathidiopsis socialis Fabre-Domergue, 1889 from the intertidal benthos of marine estuary channel between Three Mile Harbor and Gardiner's Bay. Imaged in Nomarsdki DIC on Olympus BH2S using SPlanapo 40 0.95 and SPlan 100 1.25 oil objective with oiled condenser, plus variable phone camera cropping on Samsung Galaxy S9+.
The cells measure 70 um in length. I have shown this species before, but this is the first time I imaged it under oil immersion and found individuals with few food vacuoles which in the prior observations obscured the macronucleus. Here it is clearly seen as an elongated sausage shape making certain the diagnosis of S. socialis. The cell body is ovoid to reniform and somewhat dorsoventrally flattened and shows a shallow "inpocketing" depression 1/3 to 1/2 of the way down from the apex. Again, this is the first time I was able to demonstrate that the inpocketing contains many rod-shaped toxicysts. The cell surface is sculptured into a system of longitudinally spiraling ridges and furrows. The cytostome is apical and slit like. There is a delicate cytopharyngeal basket of nematodemal rods. There is a prominent row ("pseudomembrane") of stiff toxicyst-bearing palps running from the cytostome and terminating at the inpocketing. These palps accompany and overshadow a brosse of the same length. There is a large posterior contractile vacuole. The animals tend to cluster together in the same area. Based on the size, swimming habit, prominence of the row of palps accompanying the brosse, and macronuclear shape, these features are consistent with Spathidiopsis socialis. Kahl, 1926 described this inpocketing or Gru¨bchen on its lateral surface and a “Pseudomembrane” (i.e. the row of toxicyst bearing palps adjacent to the brosse).
Lipscomb and Riordan have extensively studied and clarified the genus Spathidiopsis and its relationship with its sister taxon Placus and their confusing and convoluted taxonomy and nomenclature (1,2). "SPATHIDIOPSIS Fabre-Domergue, 1889 is a reniform, prostomatid ciliate found swimming over surfaces in marine, brackish, and freshwater habitats in search of other protists as prey. The members of this genus and its sister-taxon, Placus, are the only two genera within the family Placidae (Small and Lynn 1985). The family has been placed in the class Prostomatea (Schewiakoff 1896) and order Prorodontida (Corliss 1974) because its members have somatic monokinetids with a radial transverse ribbon, a straight nonoverlapping postciliary ribbon, and anteriorly directed nonoverlapping kinetodesmal fibril, an apical cytostome lacking complex oral cilia, a brosse, and toxicysts (Corliss 1979; Grain et al. 1978; Lynn 2008; Lynn and Small 2002; Small and Lynn 1985)" (1). Placus lacks the "pseudomembrane" of toxicyst-bearing palps seen in Spathidiopsis.
Liposcomb and Riordan redescribed and revised the family Placidae and the genus Spathidiopsis: "Genus Spathidiopsis Fabre-Domergue, 1889 Synonyms. Thoracophrya Kahl, 1926 (in part); Thoracophrya Kahl, 1928 (in part); Toracophrya Klein, 1928; Placus Kahl, 1930 (in part); Placus Curds, 1982.
Species. Spathidiopsis socialis Fabre-Domergue, 1889 (type by monotypy); Spathidiopsis buddenbrocki Sauerbrey, 1928; Spathidiopsis luciae Kahl, 1926; Spathidiopsis eforianus Tucolesco, 1962. Diagnosis. Reniform to ellipsoid placid ciliates with an inpocketing and “pseudomembrane” (i.e. row of toxicyst-bearing palps adjacent to the cytostome and brosse). The inpocketing contains many rod-shaped toxicysts. The brosse consists of a row of paired kinetosomes and a row of single kinetosomes that begins adjacent to the cytostome and continues down the ventral side one-third to one-half the length of the cell to terminate in the inpocketing. The cytostome is slit-like and surrounded by paired oral kinetosomes that lack cilia". (1).
These authors describe a wide size range for S. socialis: Spathidiopsis socialis Fabre-Domergue, 1889
Synonyms. Thoracophrya marina Kahl, 1927; Thoracophrya lucia var. livida Wang and Nie, 1932; Placus socialis Kahl, 1930; Placus salinus Dietz, 1964; Placus salinus Borror, 1972; Placus striatus Grain et al., 1978; Spathidiopsis striatus Small and Lynn, 1985; Spathidiopsis salinus Carey, 1992; Placus dogieli Burkovsky, 1970; Spathidiopsis dogieli Carey, 1992; Placus marina Foissner et al., 1994; Placus salinus Xu et al., 2005.
Description. Ovoid, 32–130 um long by 24–82 um wide. Laterally flattened. Cytostome slit-like. Spiraling kineties, 30-32, usually 31. Tends to aggregate into groups, hence the species name, socialis.
- The Ultrastructure of Placus striatus and a Revision of the Family Placidae (Ciliophora) DIANA L. LIPSCOMB and GAVIN P. RIORDAN. J. Eukaryot. Microbiol., 59(4), 2012 pp. 407–422
- A Molecular and Ultrastructural Description of Spathidiopsis buddenbrocki and the Phylogenetic Position of the Family Placidae (Ciliophora). DIANA L. LIPSCOMB, BRUNELLA M. BOWDITCH and GAVIN P. RIORDAN. J. Eukaryot. Microbiol., 59(1), 2012 pp. 67–79
Fotos / Sonidos
Observ.
closterium_mysteriumDescripción
Video: https://youtu.be/FTEfuyBeFbY
Sampling location: A water sample was taken from the bank of the Vuoksi River.
Date and time of collection: 22 Jul 2023 at 12 PM
Date and time of observation: 23 Jul 2023 at 9 PM
The sample was stored at room temperature in a plastic container.
All images and videos are of the same organism.
Taphrocampa selenura Gosse, 1887
Diagnosis:
• caterpillar-like body
• body length ~270 µm
• trophi length ~40 µm
• the mastax is virgate with strongly asymmetric trophi
• toes length ~30 µm
• foot rudimentary, toes long, slender, tapering and decurved; they are wide apart at the base and form almost a semicircle when viewed dorsally or ventrally; their length is about one-ninth of total length
• a concertina-like integument
• dorsal epidermis is sticky with transverse folds and it is abundantly covered with adherent foreign particles
• auricles retractable
• retrocerebral organ with attached eyespot
• two small symmetrically placed dorsal antennas on the head
• at least one small antenna at the posterior end, above the toes
• it appears that there are lateral antennas (?)
References:
Rotifer fauna of the USSR (Rotatoria). Subclass Eurotatoria (Orders Ploimida, Monimotrochida, Paedotrochida) (221, Fig. 99) by L.A. Kutikova (1970)
Observations by Michael Plewka https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/E-TL/Genus/Taphrocampa.html
Observation by Dr. Martin Kreutz https://realmicrolife.com/taphrocampa-annulosa/
https://www.nies.go.jp/chiiki1/protoz/morpho/rotifera/r-taphro.htm#Taphrocampa%20selenura
http://www.rotifera.hausdernatur.at/HigherTaxonomy/Index
http://rotifera.hausdernatur.at/Rotifer_data/images/addscan/_full-size/Taphrocampa%20selenura%20Gosse,%201887%20[Harring%20et%20Myers,%201924].jpg
Fotos / Sonidos
Observ.
polarblairxDescripción
Growing on a clay slope behind the Mabel Smith Douglass Library, with antheridial cups, few sporophytes
Fotos / Sonidos
Observ.
peptolabDescripción
Caenomorpha medusula PERTY, 1852 from the superficial river edge benthos of the freshwater segment of the estuarine Peconic River. Imaged in Nomarsdki DIC on Olympus BH2S using Splanapo 40 0.95 and Splan 100 1.25 oil objectives plus variable phone camera cropping on Samsung Galaxy S9+.
This metopid ciliate is a fast swimmer and often difficult to track, but also holds on to the substrate thigmotactically with the dorsally located long cirri. It is an inhabitant of saprobic anoxic decaying benthic organic debris. My population averages 100 um in total length including the single long caudal spine. There are three macronuclear nodules with two sometimes incompletely separated and a single micronucleus. There is an anterior refractile granular agggregate. The cytoplasm contains diverse rod-shaped bacterial symbionts. The contractile vacuole was rather large in diastole in some individuals. All of these features are in agreement with the most recent redescription by Li et al 2017 (1).
Li et al 2017 provide the most recent and detailed redescription of the species. In describing the most important characters, they write: "Although this species has been studied for more than 150 years, species identification remains problematic because many descriptions are based only on live materials without redescription of the ciliature. Furthermore, some features, e.g. body shape, appear to vary among individuals in different environments, but, nonetheless, have traditionally been used as key characters in the taxonomy of Caenomorpha. The original description of Caenomorpha medusula by Perty (1852) was rather superficial and failed to note some important features (e.g. the numbers of adoral membranelles, bell kineties and spines, etc.), which renders the identification of this organism difficult. However, according to the original and subsequent investigations, this species can be recognized by a combination of the following characters: (i) multiple macronuclear nodules; (ii) two unequal-length bell kineties; (iii) one conspicuous posterior spine" (1).
"Description: Body medusoid, covered with a transparent rigid pellicle, 112–125 × 50–65 um in vivo with a ratio of length to width about 2:1. Posterior spine slender, about 45–55 um long in vivo; ratio of spine length to body length about 0.4. Plump rod-shaped epibiotic bacteria often found in US population but not found in China population. Cytoplasm clear and colourless, with some dark globules (1–2 m across) and an aggregate of transparent granules in anterior body part, rod-like bacteria exist in cytoplasm in China population, very likely endosymbionts. Edges of preoral bell never adjoin closely to posterior body; peristome narrow, deep funnel-shaped. Cytostome near base of spine; undulating membrane recognizable after staining, length of membrane about 50 m long. Contractile vacuole located near base of spine, about 15–20 m in diameter, pulsates at intervals of 3–5 min. Three (41 of 60 cells [Chinese population], 36 of 45 cells [US population]), four (19 of 60 cells [Chinese population], 8 of 45 cells [US population]) or five (1 of 45 cells [US population]) macronuclear nodules, usually ovoid or ellipsoidal, arranged in line, located in center of cell, sometimes incompletely separated; one micronucleus, ellipsoidal, near macronuclear nodules. Movement leisurely, spiraling while rotating around the long axis of the body" (1).
"Two strongly thigmotactic bell kineties about 68 um and 35 um long, respectively, located in anterior part of dorsal side of cell, consist of about 94 and 56 cirri (Chinese population), and 108 and 59 cirri (US population) respectively (n = 21); cirri in each kinety arranged in indistinct zig-zag pattern. Perizonal stripe beginning near anterior end of cell, about 6.4 um wide at middle part, composed of 114–180 kineties (114–169 in China population, 123–180 in US population), spiraling 450 degrees around axis; each kinety inclined about 60 degrees to edge of shield; longest kinety (at middle of stripe) composed of about 15 pairs of kinetosomes in both populations, whereas ones near oral region with only two pairs of kinetosomes. Adoral zone composed of 41-67 membranelles (Chinese population), each with three or four rows of kinetosomes, spiraling 360 degrees around body axis from near the distal perizonal stripe, terminates near cytostome. Undulating membrane on undersurface of preoral bell (i.e. roof of peristomial region), about 50 m long. Cilia on base of spine, invariably arranged in two short kineties, each 10–15 m long, composed of about 25 kinetosomes each. The two spine kineties inclined about 20 degrees to each other, converge posteriorly" (1).
Jankowski provided an older but elegant description: "Body medusoid, covered with a clear transparent rigid pellicle that looks like an armour; body length, without a spine, is 70-90 x 65-70 ul, spine 30-35 um long. The shield bears no ciliary meridians except for those of a perizonal ciliary stripe. Instead. it bears two groups of long thick flexible cirri, with 8-10 cirri in each group: they are perfectly seen from the left side. These cirri are highly thigmotactic- one can frequently observe a prolonged adhesion of the animals to sapropelic particles by the aid of these cirri, while both perizonal cilia and adoral membranelles continue their activity. The edges of the shield never adjoin closely to the body surface; instead, a narrow deep funnel may be observed between them. The perizonal ciliary stripe occupy the margin of the shield; it is composed of 5 ciliary rows not separated into two groups, unlike that of Metopidae (where it includes 2 upper and 3 lower kinetics). The PCS of Caenomorpha looks like a wide densely ciliated field composed by a number of short oblique ciliary rows with 5 kinetosomes each. It serves for both feeding and movement in this genus. The synchronous beating of the perizonal cilia and adoral membranelles produces a rotation of the swimming animal and, in addition, creates the intense water current along the buccal groove. driving the food-particles into the intrastomium. The cytostome in C. medusula occupies a typical for all the caenomorphids posterior position with a thin tubular cytopharynx raising right up into the anterior body part, where colourless food vacuoles are concentrated" (2).
- Description of two species of caenomorphid ciliates (Ciliophora, Armophorea): Morphology and molecular phylogeny. Song Li, William A. Bourland, Saleh A. Al-Farraj, Lifang Li and Xiaozhong Hu. European Journal of Protistology 61 (2017) 29–40.
- Morphology and Evolution of Ciliophora. III. Diagnoses and Phylogenesis of 53 Sapropelebionts, Mainly of the order Heterotrichida. ANATOL W. JANKOWSKI. Arch. Protistenk. Bd. 107, S. 185-294 (1964)
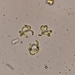
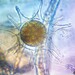
Fotos / Sonidos
Observ.
dgborinDescripción
5 specimens, but I'm not really sure they are the same species.
Fotos / Sonidos
Observ.
peptolabDescripción
Spirostomum ambiguum Ehrenberg, 1834. from the superficial river edge benthos of the freshwater segment of the estuarine Peconic River. Imaged in Nomarsdki DIC on Olympus BH2S using Splanapo 20 0.70 and SPlanapo 40 0.95 objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cell measures 960 um in length. The peristome is 2/3 the length of the body with the cytostome at the posterior 2/3 location. There is a large terminal contractive vacuole with a long collecting canal reaching almost the anterior end of the cell. The macronucleus is moniliform forming a chain of connected elongate spindle shaped macronuclear nodules beginning at the anterior 40% of the cell length and extending almost to the posterior end.
"Spirostomum ambiguum Ehrenberg, 1834. [syn: Trichoda ambiguum Müller, 1786; S. ambiguum var. major Roux, 1901] 900 m several mm long. Length: width ratio about 9-17. 15-25 kineties on each side; heterogeneous, numerous (4-5) CG rows per stripe. Peristome always longer than 1/2 of the body length, often reaching 2/3. CV much shorter than body length, rarely exceeding 1/10. The color depends on cytoplasmic granules. Moniliform MAC with 12-50 (avg. 15-25) nodules not exceeding 35 45 m in length when stained by Feulgen reaction. Numerous (up to 100) MICs 1-2 m long. Mono phyletic. Only found in freshwater. Reported in central and northern Europe, England, Russia, central Africa, USA, Jamaica, India and Japan. It sometimes harbors prokaryotic symbionts in the MAC. Spirostomum ambiguum is a well-defined, easily recognizable morphospecies whose monophyly is also strongly supported by molecular sequences" (1).
"Differential diagnosis
1) Size in vivo 1-000-4000 x 48-100 um, mostly 1200-2000 um. Visible with the naked eye as a white thread. Very flexible and contractile, shortens to approximately 390-430 x200-220 um. Contracted cells cigar-shaped.
2) Shape slender to moderately broad worm-shaped, more or less parallel-sided, 10-17 times longer than wide. Front end rounded, rear end truncated. Slightly flattened laterally. Ventral side in area of the mouth entrance slightly bulging.
3) Macronucleus moniliform or rosary-shaped, consists of 10-50, mostly 15-20 ellipsoids, about 18-53 x 12-24 um large nodes that form a long band that approaches the dorsal side. The number of nodes correlates positively with the age of the cell (REPAK & ISQUITH L974).
4) Contractile vacuole at the posterior end, with a long collecting duct extending forward along the dorsal side which sometimes shows ampullary extensions.
5) Close under the pellicle there are many spherical, spherical, yellowish granules arranged in elongated bands of 4-5 rows each which give the cell a yellowish to brownish color.
6) About 70-90 slightly spiraling rows of cilia, consisting of basal bodies arranged in pairs are constructed, but only the front one has a cilium.t
When the cells contract (startle reaction), the rows of eyelashes spiral around the body.
7) The adoral membrane zone extends from the anterior end to the posterior third (about 65-70 % of the body length) and turns to the right at the lower end. Parallel to the adoral membranelle zone a non-ciliated oral groove, which is bordered on the right by an undulating membrane.
8} Movement hatching, worm-like crawling and writhing. Fluidity with longitudinal rotation axis, with the front end describing a cone-shaped body of revolution. In the plankton falling floating with inclined longitudinal axis" (2).
"Spirostomum ambiguum is easy to recognize because of its size and shape, but the differentiation with S. minus causes considerable difficulties. There are many shape variations which occur, size and shape are not reliable distinguishing features. Usually S. minus is significantly slimmer and almost never reaches a length of 1 mm, while S. ambiguum is more compact, stockier and over 1 m long. Only the length of the adoral membrane zone in relation for body length (S. ambiguum: 65-70 %; S. minus: 35-50%) remains reasonably reliable distinguishing feature, but here too the differences do not seem to be too pronounced. Furthermore, the ciliates S. semivirescens have a similar size and shape (up to 2 mm, very slender, adoral membrane zone up to 50 % of body length, green through symbiontic algae) and Homalozoon vermiculare (up to 1.5 mm, 5-21 contractile vacuoles along the dorsal side, mouth small and only at the front end) as well as microturbellaria and nematodes. The characteristics 1,3 and 7 are particularly important for identification" (2).
- Focusing on Genera to Improve Species Identification: Revised Systematics of the Ciliate Spirostomum Vittorio Boscaroa,1, Daniela Carducci, Giovanna Barbieri, Marcus V.X. Senra, Ilaria Andreoli, Fabrizio Erra, Giulio Petroni, Franco Verni, and Sergei I. Fokin. Protist, Vol. 165, 527–541, August 2014
- FOISSNER W., BERGER H. & KOHMANN F. (1992): Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems - Band II: Peritrichia, Heterotrichida, Odontostomatida. – Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, 5/92: pp 317-25.
Fotos / Sonidos
Qué
Hongo Estrellita (Astraeus hygrometricus)Observ.
pratyekaDescripción
Seven revolute rays of the exoperidium are visible. Exoperidium interior is deeply fissured, maximum unbroken interior segment length near ray tip ≈5mm. Nearby angiosperm species providing potential host root systems include two Acacia species (at least one non-endemic), one non-endemic Grevillea and one Macrozamia communis. It is considered probable that an Acacia is the host. This could be verified after spore release is completed and the specimen is collected.
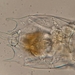
Fotos / Sonidos
Qué
Orden SessilidaObserv.
fmorales369Descripción
Sample taken from a bryophyte wash. Objective: 40x
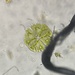
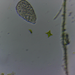
Fotos / Sonidos
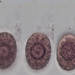
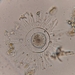
Qué
Diatomeas (Clase Bacillariophyceae)Observ.
ha300Lugar
121, Route Royale, Beau Bassin, Quatre Bornes, Port Louis District, Moka, Mauritius (Google, OSM)Qué
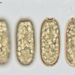
Pinos, Ocotes Y Piñones (Género Pinus)Observ.
christineyoungDescripción
Pine ovulate strobilus, 10x.
*Microscope slide of longitudinal section, stained
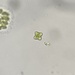
Fotos / Sonidos
Observ.
someplantDescripción
Magnification of all photos: 600×
Habitat: organic debris and decaying leaf matter from a freshwater pond. This one was observed four days after collection.
Photo taken with a Celestron PentaView Digital Microscope. According to their website, the FOV (i.e. the diagonal width) at 600× is 100 µm.
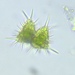
Fotos / Sonidos
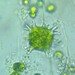
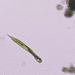
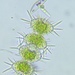
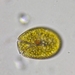
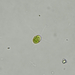
Qué
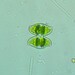
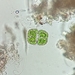
Género CarteriaObserv.
jordi_mvDescripción
Unicellular green algae with 4 flagella
400X
Eyepiece: WF10X DIN/18MM
Objective: S40/0.65 160/0.17
Sample of river water containing filamentous algae belonging to the genus Spirogyra.
Fotos / Sonidos
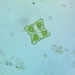
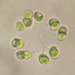
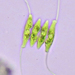
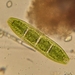
Qué
Género DiatomaObserv.
jordi_mvDescripción
400X
Eyepiece: WF10X DIN/18MM
Objective: S40/0.65 160/0.17
Sample of river water containing filamentous algae belonging to the genus Spirogyra.